OPV Cell Fabrication
| ✅ Paper Type: Free Essay | ✅ Subject: Physics |
| ✅ Wordcount: 2541 words | ✅ Published: 12 Mar 2018 |
To fabricate the inverted OPV cells with the structure of ITO/ZnO/P3HT:PCBM/PEDOT:PSS/Ag, the ITO was first coated onto a glass substrate. The ITO coated glass substrate was then cleaned by ultra-sonication in detergent solution, deionized water, acetone and isopropanol sequentially for 10 minutes each. After that, the pre-cleaned and pre-patterned ITO substrate was exposed to a UV-ozone treatment for 10 more minutes.
A solution of ZnO was prepared for spin coating onto the ITO coated glass substrate. To prepare the solution, 0.15g of ethanolamine (NH2CH2CH2OH) and 0.6g of zinc acetate dihydrate [Zn(CH3COO)2∙2H2O] were dissolved in 5 mL of 2-methoxyethanol [CH3OCH2CH2OH] with continuous stirring for 24 hours. After that, 1mL of ammonia (NH3) was added to the solution and stirred for 2 more hours. To remove the inpurities, the solution would need to be filtered through a 0.25mm PVDF filter. With the technique of spin coating at 3500 rpm for 90 s, a ZnO film of about 40 nm would be coated onto the pre-cleaned ITO coated glass substrates. Mild washing would then be performed on the ZnO films with isopropyl alcohol and it was blown dry in air.
In the next step, poly (3-hexylthiophene) (P3HT) and phenyl-C61-butyric acid methyl ester (PCBM) are mixed in the proportion of 10 : 8. The mixture was then dissolved in 1,2-dichlorobenzene solution and to be spin-coated to a thickness of approximately 200nm on the ZnO/ITO layer prepared. Annealing at 120 oC was then carried out to the film for 10 minutes.
The hole transport layer of PEDOT:PSS was then spin-coated on the bulk layer of P3HT:PCBM from a commercial solution (Clevios) under the temperature of 120 oC for 10 minutes.
In the last step, under a pressure of about 1 milliPascal, the silver electrode of a thickness about 100 nm was coated onto the PEDOT:PSS layer. The devices then needed to be annealed for 10 minutes at around 70 oC under a pressure of about 1 milliPascal.
After that, the device was cooled down to room temperature. Using a source meter at AM1.5G illumination with a solar simulator, the photovoltaic characteristics of the cell were measured. The photocurrent measurements were done by using a source meter at AM1.5G illumination. This value of photocurrent was used to calibrate the illuminator for measurements later on.
The area of the devices was 0.9 cm2.
Intro of OPV, normal and inverted geometry
In recent years, there has been great interest in organic photovoltaic cells (OPVs) due to various advantages over the conventional silicon solar cells. Some of the prominent advantages include low cost of manufacturing, light cell weight, high power conversion efficiency reaching 10% 1 and being environmentally friendly.
The structure of the photoactive layer in these organic photovoltaic cells is usually an amalgamation between a fullerene derivative as an electron acceptor and a p-conjugated polymer as an electron donor. Among different materials, poly (3-hexylthiophene) (P3HT) and phenyl-C61-butyric acid methyl ester (PCBM) have attracted wide attention for being developed to bulk heterojunction [2]. This is because of their outstanding conductivity for both electrons and holes and good stability in atmospheric conditions [3].
Aside of these advantages, a major challenge in the study of OPV cells is to combine the high power conversion efficiency and operational stability. To tackle this problem, there are two device structure set forth, namely the conventional geometry and inverted geometry.
For devices made according to the conventional geometry, as shown in Fig 1, the photoactive layer, which is usually a blend of the P3HT:PCBM, is lodged between two electrodes, such as an aluminum electrode and an indium tin oxide (ITO) electrode. Upon shining of light, the electrons and holes are photo-excited and then separated to be collected at the two electrodes. Electrons are gathered at the aluminum electrode and holes are collected at the ITO electrode.
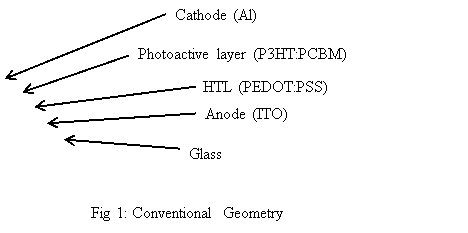

Devices with such geometry usually have relatively high power conversion efficiency. Nevertheless, the stability of such devices is compromised because these devices are easily affected by oxygen and humidity present in the atmosphere. This is due to the fact that the aluminum electrode has a low work function and it is much susceptible to oxidation [4]. Thus, the stability of devices made with the conventional geometry is restricted. However, the oxidation of aluminum electrode is not the only factor responsible for device degradation. Other factors such as: (1) charge carriers being accumulated at the interface between the photoactive layer and the electrode, (2) metal ions diffusing from the electrodes to the photoactive layer [5], (3) the photoactive layer being unstable and degrade in atmospheric conditions [6], (4) the morphology of the photoactive layer varies in the nanoscale [7]. One of the suggested ways to improve the stability of such OPV cells is to add a layer of encapsulation to prevent the oxygen and moisture from entering the devices [8]. Nevertheless, the encapsulation cannot prevent the degradation reaction from happening within the OPV cells. Therefore, the intrinsic stability of devices needs to be improved. One achievable way is to use the inverted geometry with reversed charge collection.
For OPV cells manufactured with the inverted geometry, as shown in Fig 2, the photoactive layer is middled between an electron transport layer (ETL) and a hole transport layer (HTL) which are in turn lodged between two electrodes, which are usually indium tin oxide electrode and a metal with high work function like silver. Upon photo excitation, the electrons generated will go through the ETL and be collected by the ITO electrode; the holes generated will go through the HTL and be gathered by the metal electrode [9].


The high work function of the metal electrode used makes the electrode unreactive to the oxygen and moisture present in the atmosphere, thus, a higher stability of the OPV cells can be maintained in a relatively long time period. However, the power conversion efficiency of OPV cells with inverted geometry is generally lower than those OPV cells made with conventional geometry [10].
Info on ZnO
Several materials are commonly used for constructing ETL, some examples are ZnO, LiF[11], and Ca [12]. Currenly, ZnO is most recognized as a good material for ETL because of its high electron mobility, high photo-stability, low work function and optical transparency [10]. Study done by Krebs et al. [2] has shown that inverted OPV cells with ETL made of ZnO exhibit an extended lifetime. In their study, I–V measurements were performed under illuminated conditions in both presence and absence of UV light. Their results showed that there is increased recombination when the oxygen molecules are desorbed from the surface of ZnO layer due to the photoelectrons when the cell is in operation. In the night time when the cell is not in operation, oxygen molecules are adsorbed onto the ZnO layer again from the atmosphere, thus restoring the OPV cells’ performance. This cycle of desorption and re-adsorption of oxygen molecules causes the inflection point in the I-V measurement to disappear first and re-appear later.
Other than the factors affecting the OPV cells in operation, manufacturing factors exert equally significant influence on the performance of OPV cells. One of such factors is the trap state.
In the band gap of ZnO which is a metal oxide semiconductor with nanostructure, there is presence of localized energy states, which are also called trap states. These trap states make the charge transport in ZnO to be different from the bulk material and they exert adverse effect to the charge transport in ZnO [13]. The amount of such trap states increase exponentially from the top of the valence band to the bottom of the conduction band.
According to previous research, different amount of trap states can even be present in the band gap of materials which are chemically identical. The different amount of trap states is a result of difference in crystallinity which means the perfectness of the crystal. High electron diffusivity and mobility is usually associated with materials which have higher crystallinity [14].
The crystallinity can be varied by undergone ZnO film through annealing process in different temperature, different density of trap states can thus be achieved. The devices which undergo annealing in higher temperature will have lower depth of trap states, thus consequently exhibiting a better photo-conversion efficiency and greater operational stability.
Annealing effect on ZnO, FF, Voc Isc info
To further illustrate the effect of annealing on the crystallinity of ZnO layer and consequently the effect on the OPV performance, a study was done by N. K. Elumalai et al.[1] with two devices which have their ZnO thin films annealed at two different temperatures after the ZnO film is coated onto the ITO substrate.
As shown in Fig. 3, the current–voltage measurement was done under the illuminated condition of 1 sun for both device A and device B. Device A had its ZnO film annealed at 240 oC, whereas device B had its ZnO film annealed at 160 oC.


In the current–voltage measurement, the value of the current when the voltage is zero is called the short circuit current (Isc). In the significance of an OPV cell, Isc is the amount of current going through the cell when the cell is short circuited, which means the voltage across the cell is zero. Hence, the Isc is the maximum current which can be possibly drawn from the OPV cell. The light conversion ability of the OPV cell has a direct influence on the value of Isc. Hence, Isc is useful in characterizing the OPV cell performance.
The value of the voltage at the point when the current is zero is called the open circuit voltage (Voc). Voc is the maximum voltage which can be possibly drawn from the OPV cell. In theory, the Voc is dependent either on the work function of the electrodes used or the energy difference between the highest occupied molecular orbital (HOMO) of the electron donor material and the lowest occupied molecular orbital (LOMO) of the electron acceptor material in the OPV cell, depending on whether the contact is non-ohmic or ohmic.
With the current–voltage measurement, the fill factor (FF) can be determined. Fill factor is the ratio of maximum power to the product of Voc and Isc. By going through every point on the (I–V) measurement curve, there is such a point with the current (Imax) and voltage (Vmax) values that maximize the fill factor value according to equation,

Hence, the fill factor is determined.
In addition, the power conversion efficiency (PCE), can be found from the current–voltage measurement. PCE is the ratio of the energy output from the OPV cell to the solar energy the cell received from illumination source. The energy output from the OPV cell is defined as Pcell, the solar energy the cell received is defined as Psun. The PCE can be calculated from the equations shown below.
can be found from the current–voltage measurement. PCE is the ratio of the energy output from the OPV cell to the solar energy the cell received from illumination source. The energy output from the OPV cell is defined as Pcell, the solar energy the cell received is defined as Psun. The PCE can be calculated from the equations shown below.


With reference to Fig. 3, it is obviously that device A has a better performance since it shows a higher short circuit current density and a higher open circuit voltage than device B does. With some simple calculation, the fill factor and the power conversion efficiency of both device A and device B can be determined and summarized in Table. 1.
|
Device |
Isc (mA/cm2) |
Voc (V) |
FF (%) |
PCE (%) |
|
A |
10.5 |
0.56 |
61.6 |
3.6 |
|
B |
8.2 |
0.52 |
59.8 |
2.5 |

From the Isc, Voc and PCE, it is obvious that the device A is superior that device B in terms of photovoltaic performance. Hence, it proved that annealing of ZnO film changes the crystallinity of the nanostructure, reduced trap states density and consequently lead to better photovoltaic performance.
In layer ZnO, Voc vs. T in detail examine
To examine the effect of trap states of ZnO in more details, the concept of trap depth should be introduced. The trap depth, represented by the symbol  , is the depth of the distribution of the trap states in the band gap of ZnO layer. An equation relating the trap depth and Isc is employed [15], as shown below.
, is the depth of the distribution of the trap states in the band gap of ZnO layer. An equation relating the trap depth and Isc is employed [15], as shown below.

From this equation, it is obvious that the trap depth is related to the short circuit current which in turn is a function of temperature and illumination intensity. The pre-exponential term Io in the equation summarized factors such as mobility and density of the photo-excited carriers. Researches have been done by N. K. Elumalai et al.[1] to find the effects of different temperature and illumination intensity on the Isc. By a semi-log plot, it is easy to find the trap depth at different condition of temperature and illumination intensity.

For this study, it is evident that the trap depth of device A is much lower than device B. This is in line with the theoretical prediction which says that the annealing at higher temperature make the nanostructure have a more perfect crystallinity and thus less trap states distribution.
The operational stability of these inverted organic solar cells is influenced by the trap depth and variation of the open circuit voltage. Hence, the effect of temperature and illumination intensity on Voc should be noted. There is on such equation proven to be useful as shown below

In this equation, the recombination is assumed to be Langevin type, Eg signifies the effective band gap, which is the energy difference between the highest occupied molecular orbital (HOMO) of the electron donor material and the lowest occupied molecular orbital (LOMO) of the electron acceptor material; the effective density of trap states is represented by Nc; p and n represent the hole density and electron density respectively; e and kB have their usual meaning of elementary electron charge and the Boltzmann constant [16].
From the equation above, when the temperature decreases, the Voc will approach more closely to the effective band gap. Nevertheless, this is only true under the condition that the contacts in the device are all ohmic. When contacts are all ohmic, the barriers for charge carriers are low at the interface. However, in the case which contacts are non-ohmic, charge carriers will stuck and accumulate at the interface due to the higher barrier present. Hence, the Voc will be determined by the difference in the work function of the two electrodes.
In Fig 5, the effect of temperature and illumination intensity on the Voc is illustrated. In Fig 5 (a), the ZnO layer in the device is annealed at a higher temperature, the Voc decreases almost linearly when the temperature increases for different illumination intensity. This observation can be explained by an increase in the phonon scattering in the photoactive layer and the ZnO interlayer when the temperature increases. In Fig 5 (b), Voc remains relatively constant when temperature is below 180 K for various illumination intensities, but the linear decrease of Voc is observed again when the temperature increases above 180 K; this may be due to the fact that the resistivity of the ZnO layer decreases at lower temperature. By comparing the two figures, the difference in shape can only be associated to the difference of crystallinity which is a result of annealing at different temperature. Hence, it can be suggested that the Voc is affected by the trap states at low temperature. To validate this proposition, the plot of Voc in Fig 5 (a) can be extrapolated to a temperature of 0 K, it will yield a value of 0.8 to 0.9 V. Comparing to the work function difference of the two electrodes which is around 0.7 V, this open circuit voltage is much higher, and it is indeed determined by the effective band gap.

Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal



